SNCR Technology Description
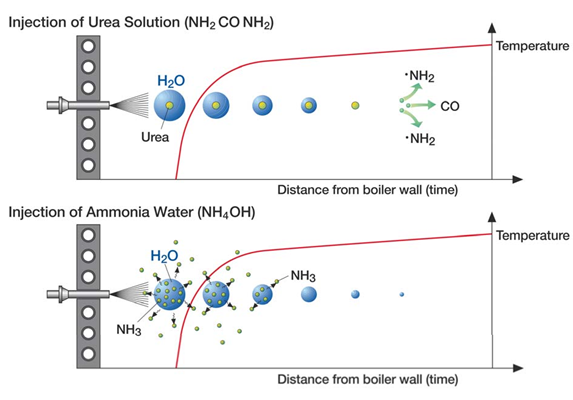
SNCR is a relatively simple chemical process. The process begins with an ammonia-based reagent, ammonia (NH3) or urea (CO(NH2)2), being vaporized either before injection by a vaporizer or after injection by the heat of the boiler. Within the appropriate temperature range, the gas-phase urea or ammonia then decomposes into free radicals including NH3 and NH2. After a series of reactions, the ammonia radicals come into contact with the NOx and reduce it to N2 and H2O.
Ammonia can be utilized in either aqueous or anhydrous form. Anhydrous ammonia is a gas at normal atmospheric temperature. It must be transported and stored under pressure. Aqueous ammonia is generally transported and stored at a concentration of 29.4% ammonia in water. At concentrations above 28%, storage of ammonia may require a per-mit, therefore some recent applications of SNCR are using a 19% solution . Decreasing the concentration, however, increases the required storage volume. Ammonia is generally injected as a vapor. Providing sufficient ammonia vapor to the injectors requires a vaporizer, even though the 29.4% solution has substantial vapor pressure at normal air temperatures. The injection system equipment for vapor systems is more complicated and expensive than equipment for aqueous systems.
2CO(NH2)2 + 6NO → 5N2 + 2CO2 + 4H2O
4HNCO + 6NO → 5N2 + 4CO2 + 2H2O
4NH3 + 4NO + O2 → 4N2 + 6H2O
In SNCR systems, a reagent is injected into the flue gas in the furnace/preheater within an appropriate temperature window. Emissions of NOx can be reduced by 30% to 50%. The NOx and reagent (ammonia or urea) react to form nitrogen and water.
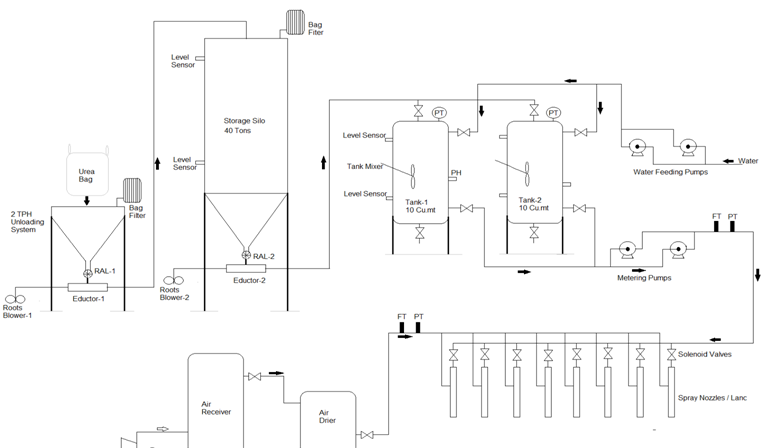
A typical SNCR system consists of reagent storage, multi-level reagent-injection equipment, and associated control instrumentation. The Reagent is sprayed into the flue gas to react with it and reduce the oxides. The temperature window for efficient SNCR operation typically occurs between 900°C and 1,100°C depending on the reagent and condition of SNCR operation.
Both ammonia and urea are used as reagents. Urea based systems have more advantages over an ammonia based system. Urea is non-toxic , less volatile and easy to store and handle safely. Urea solution droplets penetrate farther into the fluegas when injected.
Our Solution – Urea-based process
This SNCR process uses urea, CO (NH2)2 as a reducing agent. It injects an aqueous urea solution into the path of the NOx laden combustion products. The urea thermally decomposes to produce chemical species which react with NOx to form nitrogen, carbon dioxide, and water.
Equation 1 – CO (NH2)2 + 2NO + 1/2 O2 = 2N2 + CO2 + 2H2O
Equation 2 – 4NH3 + 5O2 = 4NO + 6H2O
In Equation 1 , it follows that the stoichiometric molar rate of urea relative to NO in the combustion products is 0.5, since one mole of urea potentially has two moles of NH2 available to react with NO. The urea injection process for NOx control is also temperature sensitive. The urea solution, therefore, must be injected in the temperature range of 870°-1200°C (1600°-2200°F).
Factors that influence NOx reduction efficiency
The NOx reduction efficiency of both SNCR processes depends on the following factors:
- Flue gas temperature in reaction zone
- Uniformity of flue gas temperature in the reaction zone
- Normal flue gas temperature variation with load
- Residence time
- Distribution and mixing of ammonia/urea into the flue gases
- Initial NOx concentration
- Ammonia/urea injection rate
- Heater configuration, which affects location and design of injection nozzles
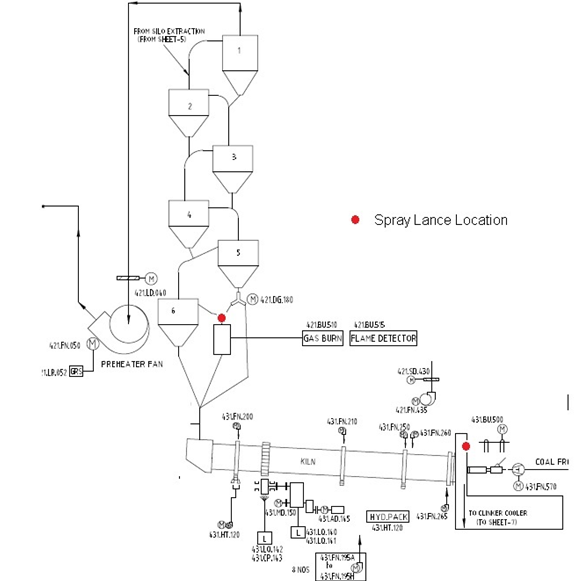
We have selected this Process based on ease of use, safety and least operating cost compared with Ammonia based NOX reduction systems. Location of the The Spray Nozzles / Lance have been so selected that it provides uniform flue gas temperature and long residence time and best mixing zone for efficient recation and highest possible NOX reduction.